|
|
Cambridge Heart, Inc. (CAMH) |
|
|
CEOCFO Current
Issue |
This is a printer friendly page! Cambridge Heart is helping to manage
the risk of Sudden Cardiac Death (SCD) with their non-invasive Microvolt T-Wave Alternans Test™ (MTWA) Cambridge Heart, Inc. Cambridge Heart, Inc., (CAMH – OTC:BB) located
in Bedford, Massachusetts, is engaged in the research, development and commercialization
of products for the non-invasive diagnosis of cardiac disease. Using innovative
technologies, they are addressing a key problem in cardiac diagnosis, the identification
of those at risk of Sudden Cardiac Death (SCD). The Company’s products incorporate
their proprietary Microvolt T-Wave Alternans Test™ (MTWA) technology and are the only
diagnostic tools cleared by the U.S. Food and Drug Administration to non-invasively
predict which individuals may be at risk of ventricular arrhythmias and SCD. All of the
Company’s products have obtained the CE mark for sale in the European community and
are generally available in most major worldwide markets. Awareness of Cambridge
Heart’s innovative products continues to increase through the publication of clinical
data from studies performed in a broad range of patients showing that those patients who
test positive for Microvolt T-Wave Alternans are at increased risk for subsequent sudden
cardiac events including sudden death. Patients at risk for sudden cardiac death now have
several treatment options available to them, including implantable defibrillators and drug
therapy. What has been missing is an accurate, noninvasive method to identify those at
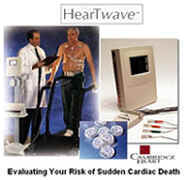
risk. Cambridge Heart now has a noninvasive product – the Heartwave – that can
detect patients at risk for sudden cardiac death who need lifesaving intervention. Conceptually what we do is simple, Mr. David Chazanovitz, president
and CEO of Cambridge Heart, Inc. explains, “We take a
look at the electro-cardiogram of the individual while under some moderate exercise. If
you have ever experienced a stress test, where electrodes are put on your chest and you
are walking on a treadmill to exercise and raise your heartbeat, this is very much what
our Microvolt T-Wave Alternans Test™ is like. We are looking for a very specific,
very minute, change in the amplitude of the T-Wave portion of the electro-cardiogram on an
every-other-beat basis. We find it with the use of our proprietary sensors, which are
placed on the body during this exercise test. The disposable sensors pass
information back into the actual product, the Heartwave, a custom computer that analyzes
the signal using our patented algorithms. When we find an individual who is Microvolt
T-Wave Alternans positive, we know we’ve found a person at risk of a sudden cardiac
event. This individual should be protected with an implantable defibrillator. If a patient
tests negative, even if they are in potentially high-risk populations with pre-existing
coronary disease, we know that this is an individual at very low risk of falling over and
dying of a sudden cardiac event.” Mr. Chazanovitz tells us about the value to
the medical community and to the public, “I have had the
pleasure of developing some interesting products that have very high medical content, that
are important to the medical community and to the patients that the medical community
serves. Microvolt T-Wave Alternans is clearly one of those products. There are three to
four hundred thousand sudden cardiac deaths in the U.S. each year. It is the number one
cause of death in individuals 45 years and older, and one in seven will die from it. Most
people fall over and die because they hadn’t been identified as being at high-risk.
If they knew they were at high-risk they could be protected with an implantable
defibrillator. The key is that they need to be easily and non-invasively identified.
That is where Cambridge Heart and MTWA come in! The disposable portion of the business is expected to
drive revenue. “This is clearly a razor, razor blade sale. We
have a piece of capital equipment, which I mentioned, called the Heartwave, which is about
the size of a laptop computer. It has a touch screen for the technician to control the
conduct of the study. The actual capital equipment is approximately $22,000 and each time
a test is performed, we sell a series of our proprietary sensors on a per-patient basis.
It costs approximately 85-95 dollars per patient. The actual capital investment is quite
modest. The cost per test is also quite modest, especially when you consider that the CPT
code for reimbursement of this test is a very attractive $426. The challenge is to get
enough equipment in the field, being used frequently enough so that the disposable portion
of the business eventually becomes the largest piece of the business,” states Mr. Chazanovitz. “In 2002 we grew our business by 38%, ending the
year at 4.3 million dollars in sales. Possibly more important, we grew our U.S. core
business approximately 56%, so we are ramping our Alternans sales,” says Mr. Chazanovitz, describing Cambridge Heart’s financial
position. “At the end of 2002 we had 3.1 million dollars of
cash, burning slightly more than a million dollars per quarter in cash. Recently we
announced that we closed on approximately $3.1 million through the sale of Series A
Preferred Stock to Medtronic and a group of private investors.. We also have the
ability to raise op to an additional $3.4 million from the same group of private
investors.” Mr. Chazanovitz addresses potential investors, “All of the fundamentals of our business and product acceptance have
never been stronger. We have just had a major presentation at the American College of
Cardiology Meeting confirming the efficacy of our product. We have wonderful partnerships
in the field with major companies. We are about to start a significant study, the MASTER
Study, where the largest of the ICD companies, Medtronic, will introduce us to fifty new
accounts enabling us to sell our equipment and get our product used both as part of the
study, as well as for clinical use within the cardiac practice. Sometimes when you go to
the newspaper and see a depressed stock price, the natural tendency is to say,
“What’s wrong with this company?” as opposed to “This is a great
opportunity, and something that I should be jumping into right now.” Needless to say,
we believe that the time is right for Cambridge Heart and that the opportunity is not yet
reflected in the share price.” |
|
To view Releases highlight & left click on the company name!
|
|