This is a printer friendly page!
RegeneRx Biopharmaceuticals is
developing Thymosin Beta 4 (Tß4), a ground-breaking drug treatment for chronic wounds

Healthcare
Biotechnology & Drugs
(OTCBB: RGRX)
RegeneRx Biopharmaceuticals
3 Bethesda Metro Center, Suite 700
Bethesda, MD 20814
(301) 961-1992

J.J. Finkelstein
President and
Chief Executive Officer
Interview conducted by:
Lynn Fosse
Senior Editor
CEOCFOinterviews.com
July, 2003
BIO:
J.J. Finkelstein, President & CEO RegeneRx Biopharmaceuticals
J.J. Finkelstein - Mr. Finkelstein is the Company's President and CEO and a member of the
Board of Directors. He provides expertise in the creation, implementation, and financing
of RegeneRx's business strategy. Mr. Finkelstein has been a chief executive officer and
consultant in the bioscience industry for the past twenty years, having served as Chief
Executive Officer of three biomedical companies since 1982, including as CEO of the
Company from 1984 to 1989 and as Vice-Chairman from 1989 to 1991. He is experienced in a
start-up environment, has been responsible for the regulatory approval and marketing of a
number of medical products in the U.S. and abroad, and has raised over $38 million in
capital to finance these ventures. He currently serves on the Boards of several bioscience
firms, including MdBio, Inc. a not-for-profit Maryland organization whose mission is to
support bioscience development and education in the State of Maryland. Mr. Finkelstein
received a business degree from the University of Texas where he majored in finance.
Company Profile:
RegeneRx Biopharmaceuticals (OTCBB: RGRX) is developing a proprietary product, Thymosin
ßeta 4 (Tß4), for the healing of chronic wounds caused by disease, abrasion, or other
pathology. For the past several years, the National Institutes of Health (NIH) has
conducted a research program to evaluate Tß4 as a wound healing and anti-inflammatory
agent. The result of this research has led to the filing of world-wide patent applications
related to Tß4.
Wounds fall into several
categories depending on whether they are chronic or acute, or result from disease, the
environment, accidents, or medical procedures. The Company has identified several
significant potential markets for Tß4: Chronic bed sores, Diabetic ulcers (usually
lower extremities), Wounds arising from genetic defects, Inflammatory eye injuries, Cuts
and abrasions, Burns (heat and chemical) and Anti-inflammatory responses formed after
laser skin resurfacing.
In February 2001, the Company
secured an exclusive worldwide license from NIH relating to all intellectual property
covered under the patent applications. Under a research agreement with The George
Washington University, the Company has exclusive rights to patents obtained from sponsored
research associated with septic shock and associated syndromes including acute respiratory
disease syndrome (ARDS). Since 1999, the Company has filed numerous additional
patent applications related to Tß4.
The Company's research and
development effort is primarily focused on collaborating with NIH and the sponsorship of
human clinical trials. Pre-clinical testing must be conducted to evaluate the potential
efficacy and the safety of an investigational drug prior to its use in human clinical
trials. The results of these studies are submitted to the U.S. FDA as part of an IND,
which must be reviewed and approved before clinical testing can begin. Clinical evaluation
involves a three-stage process. In November 2002 RegeneRx filed its first IND for Tß4,
which it received in December 2002. The Company began Phase I clinical trials in the first
quarter of 2003.
CEOCFOinterviews:
Mr. Finkelstein, please give us a brief history of RegeneRx Biopharmaceuticals and what
products you are currently developing.
Mr. Finkelstein: “RegeneRx was founded in 1982 by Dr.
Allan Goldstein, who is the Chairman of Biochemistry and Molecular Biology at the George
Washington University Medical School in Washington D.C. It is one of the oldest
biotechnology companies still in existence, having been around for about 21 years. The
first product developed by the company was Thymosin alpha 1 (T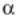 1), which was licensed out and then sold
to another pharmaceutical company. It is on the market in 31 countries and currently is in
Phase III trials in the United States. RegeneRx is currently working on a product called
Thymosin Beta 4 (Tß4), a unique molecule that has been studied quite extensively at the
National Institutes of Health (NIH) and universities around the United States as a
treatment for chronic dermal wounds as well as ocular wounds. We are currently
developing that product for those indications and taking it into human clinical
trials.” 1), which was licensed out and then sold
to another pharmaceutical company. It is on the market in 31 countries and currently is in
Phase III trials in the United States. RegeneRx is currently working on a product called
Thymosin Beta 4 (Tß4), a unique molecule that has been studied quite extensively at the
National Institutes of Health (NIH) and universities around the United States as a
treatment for chronic dermal wounds as well as ocular wounds. We are currently
developing that product for those indications and taking it into human clinical
trials.”
CEOCFOinterviews:
Why have you chosen that particular product and are other people testing it as well?
Mr.
Finkelstein: “We chose Tß4 because Dr. Goldstein discovered this product as
he did Thymosin alpha 1. It thus became one of the company’s proprietary products.
The NIH became interested in it when some data was published showing that it
sequesters or regulates a protein called Actin, which, among other things, is necessary
for cells to change shape and move throughout the body. The NIH then began a program where
they studied it in various small animal models in the lab to see how it performed in the
healing of chronic wounds and have extensively published their results. We are very
familiar with the molecules and became quite interested when we saw the results. We
in-licensed certain rights from the NIH because of our desire to commercialize this drug.
This work has been our focus for the last three and a half years.”
CEOCFOinterviews:
How far have you gotten in this process?
Mr.
Finkelstein: “We acquired the license and began doing the substantial
preclinical work, which is required before filing an IND (Investigational New Drug)
application. That took us several years and several millions of dollars. We filed for an
Investigational New Drug application with the FDA in November of 2002, and we received an
IND in December. The Phase I clinical trial started in March and is due to be completed in
August. We will evaluate the data and do all the necessary regulatory filings to begin the
next phase.. We will then file the Phase II IND with the FDA sometime in the following
month or two.”
CEOCFOinterviews:
What is the treatment and what does Tß4 do that is better?
Mr.
Finkelstein: “The current treatment for most wounds is “good wound
care,” which means cleaning the wounds, keeping them moist and bandaging them if
necessary. If there is an associated infection, topical or systemic antibiotics may be
administered, but those treat the infection, not the wound. To my knowledge, the only
product that has been approved for any chronic wound is a growth factor called RegranexTM
that has been put on the market by Johnson & Johnson, specifically for chronic
diabetic foot ulcers and with somewhat limited results. The market itself is segmented
because there are many different types of chronic wounds and is desperately looking for
products that can help promote and accelerate the healing of these wounds. Wounds become
chronic for various reasons, but some of the reasons, as opposed to acute or short-term,
are that people who are older often do not heal very well. Or, if they have underlying
diseases such as diabetes, they may have the same problem. Similarly, they may have a
genetic or autoimmune disorder, or they may be on steroids; all these factors can readily
affect the ability of the wound to heal. The market needs products that can help
accelerate the healing of wounds. In animal models that are designed to mimic the human
condition, Tß4 has shown to be quite effective in the accelerating the closure of these
wounds.”
CEOCFOinterviews:
How does it do that?
Mr.
Finkelstein: “Tß4 regulates a protein in the body called Actin, which is the
protein responsible for the movement of cells throughout the body, specifically non-muscle
cells. When you have a wound, its leading edge should have an abundance of Actin and Tß4.
However, if not, the wound may worsen and become chronic. Our interest is in restoring
Tß4 to the wound to allow Actin to do what it is supposed to do by regulating it. This
regulation of Acting, which is called polymerizing and depolymerizing, causes cells to
move, aggregate and accumulate around the wound and start the closure process. Many people
assume Tß4 is a growth factor but it is not; it operates on a very different level.”
CEOCFOinterviews:
Is it applied topically?
Mr.
Finkelstein: “Tß4 is applied topically for dermal wounds, however in the NIH
studies, it was applied both topically and through injection, and the results seem to be
similar through both routes of administration. That made us very interested in its
potential for treatment for internal wounds associated with immunodeficiency disorders
such as Colitis or Crohn’s disease where patients have constant inflammation and
erosion of the bowel that is very difficult to heal and where substantial bleeding and
pain often occur. While Tß4 is a single molecule, we refer to it as a platform technology
because there are so many different ways that it could be used, externally and internally,
to treat chronic wounds. There are many possibilities but we are a small company and
cannot pursue all of those ourselves. Consequently, we have been interested in working
with potential partners in other areas that we are not independently developing
ourselves.”
CEOCFOinterviews:
Why did you make the decision to do the application as it relates to the ocular side?
Mr.
Finkelstein: “During the NIH studies, there was an ophthalmologist at the NIH
who was very interested in ocular injury. He said, “if it works in the dermal area,
let’s see what happens in the eye”. So they did a number of studies with
laboratory animals and saw that when the eye was inured, applying Tß4 topically
substantially accelerated the healing process. There are substantial data, as well as
pictures, that show how much better and more rapidly the cornea of the eye healed with an
application of Tß4. There are a number of diseases, injuries and surgeries that can occur
in the eye that lead to wound of its surface, where more rapid or better healing would be
highly beneficial.”
CEOCFOinterviews:
What are the steps before this is commercialized and do you have plans for how that will
go?
Mr.
Finkelstein: “There are many steps, and we have a very in-depth strategic plan
of how we will commercialize the product, not only implementing the processes, but also
financing each segment. Developing any medical product clearly requires substantial
capital. We have been raising capital since I became involved in this project
approximately three and a half years ago. We have a pharmaceutical partner that has taken
an equity investment in our company twice now and we are looking at raising our next round
of capital shortly. By the end of this year, we should have enough capital to complete
several Phase II studies in the United States as well as operating capital for two to
three years. Given that the capital is available, the question is, ‘how we will
pursue the development of the product.’ We are independently developing Tß4 in the
U.S. for dermal wounds and will do so as long as possible to create as much value as
possible. We are talking to a number of companies regarding potential strategic
partnerships for other areas of use outside dermal wounds, and in other territories. We
are looking to have a several pronged approach; independently and through partnerships
working in parallel fashion.”
CEOCFOinterviews:
Is this patented?
Mr.
Finkelstein: “We have two issued patents and approximately ten additional
patents that have been filed around the world related to the product, its composition and
its use.”
CEOCFOinterviews:
How large is the potential wound-healing market?
Mr.
Finkelstein: “The figures for the wound healing market estimate it at greater
than seven billion dollars annually in the United States, which means that it is three
times that if you consider other potential markets around the world. It is a highly
fragmented market place, in that there are many different types of wounds and many ways
that people deal with the healing of wounds. It is an under-served market and many
companies have been looking for years to find appropriate drugs to use to treat people
that have chronic wounds. The fact is that the market continues to grow because of the
increase in the elderly population and the number of cases of diseases such as diabetes,
which impair healing. Any company that develops products to serve that market will have an
excellent opportunity for financial success.”
CEOCFOinterviews:
Assuming the trials progress well, what are the challenges that you face in the whole
plan?
Mr.
Finkelstein: “There are several challenges we face as the trials progress. One
is capital because these things take a lot of it, as the clinical trial process is very
expensive. We will have to be able to finance our way through it, which we have done
successfully thus far, and I anticipate with success, our ability to continue to do so.
The second thing is that when we get to the point that we have shown the efficacy of Tß4
, we will have to structure a deal with a large pharmaceutical partner interested in this
area, which must be a good deal for them and for us. We have not entered into any
agreements as yet, even though we have had a number of discussions. Our objective is
to create and build value while reducing risk, We feel that if our clinical program is
successful, we will be able to make a better deal for stock holders, although there are
obviously risks throughout the process. The third issue is competition. There are many
companies looking for new and better products that can do the things we are trying to do,
so there is always competition out there and it can pop up at any time.”
CEOCFOinterviews:
Why should potential investors be interested, and what should they know that they might
not realize when they look at the company?
Mr.
Finkelstein: I think this is the most exciting period for our company because we
have completed a substantial amount of preliminary work and now we are into human clinical
trials, and that is what makes or breaks a company. Anyone interested in us now is
coming in at a more mature stage, a stage where they are going to find out in the next
eighteen to twenty-four months how effective Tß4 may be in a human setting. This is very
exciting and offers a lot of reward if a product is successful in clinical trials.
However, I think it is important for most people to know that it is difficult to predict
success because if a drug works in animals, it does not mean it will work in humans. If it
works in a Phase 1 trial, it does not mean it will work in Phase 2, and if it works in
Phase 2, it does not mean it will work in Phase 3, although each successful step in the
process reduces the risk. I think today’s investor understands that concept a lot
better than investors did five years ago, and I know they understand more today than they
did fifteen years ago.”
CEOCFOinterviews:
In closing, what would you like people to remember about RegeneRx?
Mr. Finkelstein:
“Well, your question makes it sound like we’re at a funeral, but I think of us
as in our teen years of development – full of excitement, still some unknowns, but on
our way to adulthood! I think that this is an exciting opportunity; we certainly
believe in what we are doing. The data to date have been substantial and published in
well-known scientific journals by independent and prestigious research institutions.
RegeneRx has operated very efficiently and effectively so far. We are rapidly moving
forward and have accomplished everything we publicly said we would accomplish over the
past three and one half years, and are excited about the coming twenty-four months.”
disclaimers
© CEOCFOinterviews.com – Any reproduction or
further distribution of this article without the express written consent of
CEOCFOinterviews.com is prohibited.
|





