
© CEOCFO Magazine -
CEOCFO Magazine, PO Box 340
Palm Harbor, FL 34682-
Phone: 727-
Email: info@ceocfocontact.com


Search




Business Services | Solutions
Medical | Biotech
Cannabis | Hemp
Banking | FinTech | Capital
Government Services
Public Companies
Industrial | Resources
Clean Tech
Global | Canadian
Lynn Fosse, Senior Editor
Steve Alexander, Associate Editor
Bud Wayne, Marketing
& Production Manager
Christy Rivers -








Spark Biomedical’s Wearable Neurostimulation Device Alleviating the Symptoms of Opioid Withdrawal
 Daniel Powell
Daniel Powell
CEO
Spark Biomedical
Contact:
Spark Biomedical
844-
Interview conducted by:
Lynn Fosse, Senior Editor
CEOCFO Magazine
Published – January 25, 2021
CEOCFO: Mr. Powell, what is the overall concept behind Spark Biomedical?
Mr. Powell: We developed a wearable neurostimulation device that patients going through opioid withdrawal can use to alleviate the symptoms associated with withdrawal.
CEOCFO: What have you developed so far?
Mr. Powell: As of Saturday, January 2nd, this is an FDA cleared device for market launch, meaning it’s a fully FDA compliant device and able to be commercialized. We ran a Level 1 clinical trial out of Austin, Texas, where we tested twenty-
What that really looks like is, someone in moderate withdrawal with gut cramps and sweating and goosebumps and aches and pains and nearly in the fetal position on the ground. In one hour, they are either in mild withdrawal or have no clinical withdrawal symptoms. In just one hour of treatment, they are able to manage what is otherwise a very painful detox process.
CEOCFO: Would you tell us how your device works and why it works?
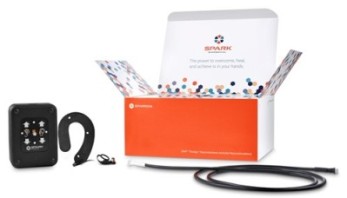 Mr. Powell: The device sits around the ear and stimulates cranial nerve endings that come up to the skin. Two specific nerves, the trigeminal and vagus nerves, are connected to structures in the center of the brain. We are able to put a mild amount of electricity similar to a TENS unit, and send signals down those nerves. They go to the center part of the brain, and they have two simultaneous effects. One effect is that they create what we call an autonomic balancing effect reducing the “fight or flight sensation that drives fear and anxiety. This helps someone have the ability to think rationally and think, “I am okay, this is okay, I am not afraid of this.” That is because opioids rewire the brain in a way that creates a dysfunction in the portion of your brain that does not allow you to process fear and anxiety easily.
Mr. Powell: The device sits around the ear and stimulates cranial nerve endings that come up to the skin. Two specific nerves, the trigeminal and vagus nerves, are connected to structures in the center of the brain. We are able to put a mild amount of electricity similar to a TENS unit, and send signals down those nerves. They go to the center part of the brain, and they have two simultaneous effects. One effect is that they create what we call an autonomic balancing effect reducing the “fight or flight sensation that drives fear and anxiety. This helps someone have the ability to think rationally and think, “I am okay, this is okay, I am not afraid of this.” That is because opioids rewire the brain in a way that creates a dysfunction in the portion of your brain that does not allow you to process fear and anxiety easily.
Most patients will tell you that they think they are afraid they will die going through withdrawal. So, we create this balancing effect that allows a patient to get the anxiety irrational fear under control. However, simultaneously, the electricity drives the production of natural endogenous endorphins, of which opioids are an exogenous endorphin, essentially. It jump-
CEOCFO: Does it work the same for everybody? Is it a standard time or voltage?
Mr. Powell: That is a great question. Everyone is definitely different. We had one patient that it just did not work on for whatever physiology. Medicine is like that. Science is like that. There are variabilities. However, I think about sixty percent of the patients in our study were also going through alcohol withdrawal. Those with alcohol withdrawal and opioid withdrawal may have a different effect, although it was still a very good effect. The difference is those who were going through just opioid withdrawals tended to have even better outcomes.
We saw some variability’s as you always do in clinical data, but nothing we could really tie it to, like men having a different response than women. It is just different bodies and different durations on how long, how many years you have been taking opioids, and different opioids. Heroin is, strangely, easier to get through, we saw, whereas methadone binds to the opioid receptors and takes a much longer duration, and it is harder to get through the withdrawal. There are several variables in the whole thing that could cause one person to respond better or worse than another.
CEOCFO: Why is it called the Sparrow Therapy System?
Mr. Powell: We tried a bunch of different names over a year ago and decided to hold a naming contest within the team. Sparrow was the that one came out on top! We liked the idea of a bird soaring free and that it was a little bird who could do mighty things. It just resonated with us.
CEOCFO: How are you introducing the system to people who should know about it?
 Mr. Powell: As a brand-
Mr. Powell: As a brand-
We know that this is a very vulnerable patient population, and we just want to serve them as best as possible. However, we are in every bit of a hurry to try to get this out to the people. I will say, just as a side note, that there is a huge need right now. We think that 2020 is going to have a fifty percent increase in opioid overdoses over 2019, and 2019 had a pretty big leap over 2018. 2018 was the first year that the death rate actually went down a little bit. However, it then spiked back up in 2019, and then COVID is just the perfect storm for causing relapse in overdoses. Being isolated, losing hope, the fear of losing a job, these are all relapse triggers. The epidemic wrapped in a pandemic has taken a real huge death toll in America that is not being talked about very much.
CEOCFO: What is the competitive landscape? Has anything like this been tried before?
Mr. Powell: From a pharmaceutical standpoint, there are a couple of what we will call comfort medicines. None of them have nearly the profound effect that you get in just one hour. Over a third of our patients had no withdrawal symptoms in one hour. There is no medication that we are aware of that does that, unless it is an opioid, unless you are literally taking an opioid substitute, which in this case we do not count.
We really got our inspiration for this solution is from acupuncture and electro-
CEOCFO: Are you funded for your next steps?
Mr. Powell: Yes. We are actually completing some now. We are doing really well on the fundraising front and are able to put that money towards growth, so yes.
CEOCFO: How are you able to get on top of funding when so many others cannot seem to get that done?
Mr. Powell: I will admit, this is the first time I have ever raised funds in my life! I watched a lot of YouTube videos on different mechanisms. However, the team we assembled has such heart, and passion, and in-
The first place I started was calling colleagues. I worked in the neuro stim field for nearly twenty years, mostly implantable products, deep brain stimulation or spinal cord stimulation, or vagus nerve stimulation. I was amazed that many of my old colleagues and coworkers in the industry were really excited to invest. Even a CEO that I used to report to invested, which is humbling and exciting and nice when that happens. He has been a huge champion of this company. I thought that fundraising was going to be the hardest part, and it has gone really smoothly.
CEOCFO: Are there other applications for what you have developed?
Mr. Powell: There definitely are. One application that is very similar that we are pursuing is to use this in babies born dependent on opioids. You have a mother who ingested opioids while pregnant, in utero; the baby is born and goes through a horrible withdrawal. It just shakes and cries and screams.
We actually just completed our first eight-
CEOCFO: You also have the Roo™ System for neonatal; does that also have an FDA clearance?
Mr. Powell: Our Roo™ System, received something called FDA breakthrough device designation this past December. However, it has not been approved to be commercialized. The breakthrough device designation is a program within the FDA to fast-
It is a great program, especially for us, to get this baby product to market as quickly as possible. You can imagine the risk profile for what is most likely an underweight, premature baby, often with other health complications. It is a different risk profile, so the study is just not a one for one application, compared to the adult device.
CEOCFO: It seems almost too good to be true, that you could stop withdrawal in an hour. Are you concerned that there will be skepticism?
Mr. Powell: There always should be, right? We even see this with vaccine trials and everything. But you want to see good science. That is what we founded this company on, which is running good credible science the correct way and doing this all in a way to demonstrate that it works with high ethics. We do not over-
We’re not resting on the one clinical study we ran once. We are going to run more studies as we continue to invest in the science, because we are nowhere near where we want to go with this. We think we can continue to improve the outcomes for patients. We only studied a five-
CEOCFO: What is the takeaway about Spark Biomedical?
Mr. Powell: It is an exciting time, where we are bringing years and years of understanding of neurostimulation to a new product. But for the first time, it does not have to be implanted in the body. It can be worn and can be used for many new applications. There are companies like ours who are willing to take the time and do the science to bring real improvement to people’s lives, so for us, it is a very exciting time!
Spark Biomedical, Daniel Powell, Opioid Withdrawal Treatment, Neurostimulation Therapy, Spark Biomedical’s Wearable Neurostimulation Device Alleviating the Symptoms of Opioid Withdrawal, CEO Interviews 2021, Medical Companies, Neurostimulation Wearable Device, Neonatal Opioid Withdrawal Solutions,Transcutaneous Auricular Neurostimulation for Opioid Withdrawal Relief, tAN Therapy, The Sparrow Therapy System, The Roo Therapy System, drug-
“It is an exciting time where we are bringing years and years of understanding of neurostimulation to a new product. But for the first time, it does not have to be implanted in the body. It can be worn and can be used for many new applications. There are companies like ours who are willing to take the time and do the science to bring real improvement to people’s lives, so for us; it is a very exciting time!” Daniel Powell